Brief on PLC Validation and Compliance
Hitendrakumar Shah
4:57:30
Description
A brief understanding and compliance to PLC Validation for Pharmaceuticals
What You'll Learn?
- You will understand brief about HMI. PLC and SCADA. Different validation strategies.
- How Agile approach and water fall approach is used
- Developing the validation skills,You will able to understand PLC validation strategies.
- What is QTP testing and manual testing
Who is this for?
What You Need to Know?
More details
DescriptionThe participants of the course will understand the common errors made related to cGXP computerized systems. The course consists of different recorded lectures.
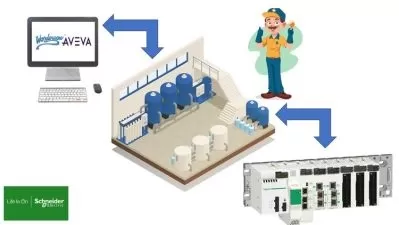
The first lecture is designed to teach the basics about HMI, PLC, and SCADA. This lecture includes a brief understanding of HMI, Industrial Usage of HMI, Benefits of HMI, a Brief understanding of PLC, Major considerations of URS in PLC, a Discussion on drawbacks of PLC, a Brief understanding of SCADA and Components of SCADA.
A Separate lecture on the basics of computerized system validation. This lecture briefly includes the importance of the topic, Introduction to Pharma 4.0, Purpose and Scope, GAMP5, Categories of Hardware and software, Validation strategies, Understanding URS, FRS, FAT, SAT, IQ, OQ, PQ, UAT, Validation plan Vs Validation Master Plan- VR Vs VMR and Major changes in GAMP 5.
The lecture on PLC validation part (Session)-1 is designed to include a brief introduction of the concept, PLC, validation strategy, Types of GXP computerized systems, Requirements for Validation, Understanding Traceability Matrix, Elements of Functional Risk Assessment (FRA), Infrastructure Risk Assessment, Process Control System validation requirements, Identifying GXP and non-GXP systems, Criticality based on system complexity and Validation of PLC logic.
The last lecture on PLC validation Part (Session)-II is designed to include a quick recap from Session – 1, an Understanding of different test strategies, a Brief on white box and black box testing, Agile testing approach during PLC validation.Difference between the Agile Approach and Waterfall approach, Verification vs. validation, QTP (Quick Test Professional) and Manual testing, Stages of Manual testing, and System Retirement Plan
Who this course is for:
- Professionals of pharmaceutical industry, Quality Assurance, Quality control (Analytical laboratory), Pharma IT, Lab Compliance, production, engineering, R&D, development, investigation team, compliance team etc.
- The persons involved in review and approval of cGXP computerised system and its validation, PLC validation and controls etc.
The participants of the course will understand the common errors made related to cGXP computerized systems. The course consists of different recorded lectures.
The first lecture is designed to teach the basics about HMI, PLC, and SCADA. This lecture includes a brief understanding of HMI, Industrial Usage of HMI, Benefits of HMI, a Brief understanding of PLC, Major considerations of URS in PLC, a Discussion on drawbacks of PLC, a Brief understanding of SCADA and Components of SCADA.
A Separate lecture on the basics of computerized system validation. This lecture briefly includes the importance of the topic, Introduction to Pharma 4.0, Purpose and Scope, GAMP5, Categories of Hardware and software, Validation strategies, Understanding URS, FRS, FAT, SAT, IQ, OQ, PQ, UAT, Validation plan Vs Validation Master Plan- VR Vs VMR and Major changes in GAMP 5.
The lecture on PLC validation part (Session)-1 is designed to include a brief introduction of the concept, PLC, validation strategy, Types of GXP computerized systems, Requirements for Validation, Understanding Traceability Matrix, Elements of Functional Risk Assessment (FRA), Infrastructure Risk Assessment, Process Control System validation requirements, Identifying GXP and non-GXP systems, Criticality based on system complexity and Validation of PLC logic.
The last lecture on PLC validation Part (Session)-II is designed to include a quick recap from Session – 1, an Understanding of different test strategies, a Brief on white box and black box testing, Agile testing approach during PLC validation.Difference between the Agile Approach and Waterfall approach, Verification vs. validation, QTP (Quick Test Professional) and Manual testing, Stages of Manual testing, and System Retirement Plan
Who this course is for:
- Professionals of pharmaceutical industry, Quality Assurance, Quality control (Analytical laboratory), Pharma IT, Lab Compliance, production, engineering, R&D, development, investigation team, compliance team etc.
- The persons involved in review and approval of cGXP computerised system and its validation, PLC validation and controls etc.
User Reviews
Rating
Hitendrakumar Shah
Instructor's Courses
Udemy
View courses Udemy- language english
- Training sessions 5
- duration 4:57:30
- Release Date 2023/12/12