The Simplest Guide to Clinical Trials Data Analysis with SAS
Aslam Khan, PgMP, PMP, SAFe, SAS
1:59:09
Description
Step into the world of Pharmaceutical industry |Clinical Trials |Clinical Research |Biostatistics |Data Management| SAS
What You'll Learn?
- Get introduced to the Life Science/ Pharmaceutical industry in simple, visual pictures
- Learn the fundamental concepts of Clinical Drug Development / Clinical Trials processes
- Understand the various Phases of Clinical Trials in the context of SAS programming
- Get introduced to the various clinical study documents like Protocol, Statistical Analysis Plan etc.
- Understand raw data and how it is collected, stored, analysed and reported
- Work hands-on with sample study data that you will import, prepare, restructure and visualize
- Generate an actual Clinical Study Reports from the derived data you will create
- See for yourself how SAS programming is an integral part of putting a drug into the market
- A guide to the SAS Certified Clinical Trials Programmer Using SAS®9 Certification Exam
Who is this for?
More details
DescriptionThis course gives an introduction to the Pharmaceutical/ Life Science industry in a simple and visual style that is easy to understand. It shows how SAS is used as a tool to work with the vast amount of clinical data within this industry. The course takes you through an example clinical study sample data, and generate various Clinical Study Reports that are submitted to the FDA (in the US) or other regulatory authorities in other countries. You will not only hone your SASÂ programming skills but will also learn essential concepts needed to work in the Pharma industry in the areas of Biostatistics and Clinical Data Management.
After the introduction to the pharma industry and learning relevant concepts about Clinical Trials, the course takes you through a hands-on training exercise to build the very important and fundamental Clinical Study Report called the Demographics Table.
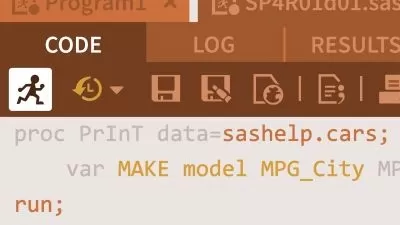
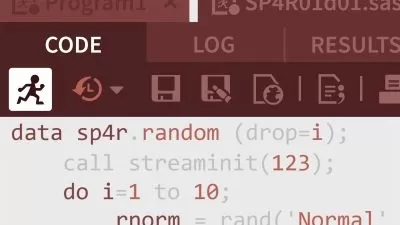
You will begin with a sample clinical study data in an Excel sheet, then you will import it into SAS, derive all necessary variables as shown in the mock table, and finally generate a clinical study report. All this will be done using guided SASÂ Programming steps with detailed explanations at every step of programming. At the end of this course, you will have learnt to work with Clinical Study Data, generate a real Clinical Study Report, and extend those steps to build other reports that constitute Clinical Trial submissions to the regulatory bodies.
Prerequisites:Â You will need basic SASÂ programming knowledge to work with code discussed in this course. You can take my course on SASÂ programming ("The Simplest Guide to SASÂ Programming") available on Udemy.
A guide to the following SASÂ certification exam:
SAS Certified Clinical Trials Programmer Using SAS®9
Earn a Certificate
When you finish listening to all videos, assignments, quizzes and practice exams, you'll earn a Certificate that you can share with prospective employers and your professional network.
Who this course is for:
- Anyone wanting to get into the Life Science / Pharmaceutical industry and looking to work in this field
- Want to take up a job as a Clinical SAS Programmer in a Pharma company or a CRO (Contract Research Org.)
- Want to just play with data using the SAS Programming concepts
This course gives an introduction to the Pharmaceutical/ Life Science industry in a simple and visual style that is easy to understand. It shows how SAS is used as a tool to work with the vast amount of clinical data within this industry. The course takes you through an example clinical study sample data, and generate various Clinical Study Reports that are submitted to the FDA (in the US) or other regulatory authorities in other countries. You will not only hone your SASÂ programming skills but will also learn essential concepts needed to work in the Pharma industry in the areas of Biostatistics and Clinical Data Management.
After the introduction to the pharma industry and learning relevant concepts about Clinical Trials, the course takes you through a hands-on training exercise to build the very important and fundamental Clinical Study Report called the Demographics Table.
You will begin with a sample clinical study data in an Excel sheet, then you will import it into SAS, derive all necessary variables as shown in the mock table, and finally generate a clinical study report. All this will be done using guided SASÂ Programming steps with detailed explanations at every step of programming. At the end of this course, you will have learnt to work with Clinical Study Data, generate a real Clinical Study Report, and extend those steps to build other reports that constitute Clinical Trial submissions to the regulatory bodies.
Prerequisites:Â You will need basic SASÂ programming knowledge to work with code discussed in this course. You can take my course on SASÂ programming ("The Simplest Guide to SASÂ Programming") available on Udemy.
A guide to the following SASÂ certification exam:
SAS Certified Clinical Trials Programmer Using SAS®9
Earn a Certificate
When you finish listening to all videos, assignments, quizzes and practice exams, you'll earn a Certificate that you can share with prospective employers and your professional network.
Who this course is for:
- Anyone wanting to get into the Life Science / Pharmaceutical industry and looking to work in this field
- Want to take up a job as a Clinical SAS Programmer in a Pharma company or a CRO (Contract Research Org.)
- Want to just play with data using the SAS Programming concepts
User Reviews
Rating
Aslam Khan, PgMP, PMP, SAFe, SAS
Instructor's Courses
Udemy
View courses Udemy- language english
- Training sessions 36
- duration 1:59:09
- Release Date 2022/12/11