Risk Management Masterclass [2023] | Certification Course
Anil Sharma Kandel
4:31:23
Description
Be an expert in ISO 14971 (RM) and ISO 13485 (QMS). Create risk assessment docs like DFMEA, HAZOP, HACCP from scratch
What You'll Learn?
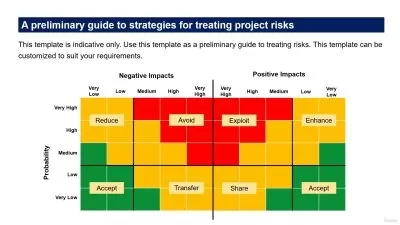
- Become an expert in creating Risk Management documents from scratch, such as PFMEA, DFMEA, HAZOP, HACCP for any enterprise.
- Learn-depth on how to perform Risk Assessment, Risk Evaluation, Risk Measurement, Risk Control and Benefit-Risk Analysis!
- In-depth understanding of Regulatory standards for GMP environment such as ISO 14791:2019, EU 2017/745 MDR, 21 CFR 820, SOR/98-282, ISO13485 and ISO 22000
- Be proficient in what Food and Drug Administration (FDA) auditors are looking for while doing Quality audits in medical device and food industry,
- Learn why CAPA, Non conformances, IQ/OQ/PQ, complaint handling process are crucial for medical device industry
- Learn in-depth about Quality Management system (QMS) regulations from different countries around the world
- How Risk management Activities is essential in creating a good manufacturing practice (GMP) environment.
Who is this for?
What You Need to Know?
More details
DescriptionThe most extensive course on Risk Management available in Udemy!
This course is designed to teach you everything that you need to know to become a Subject Matter Expert (SME) in Risk Management and Quality Management System (QMS) within Medical device, pharmaceutical industry and any other enterprise that conducts risk management activity.
Regardless of your current skills and experience, after taking this course, you will be comfortable communicating to experts in medical device industry about Quality management systems (QMS) and risk management (RM)Â standards and regulations.
By the end of this course you will be a pro at creating complex Risk Assessment and Risk Measurement documents such as PFMEA, DFMEA, HACCP, HAZOP, Benefit-Risk Analysis, Post Market Surveillance Report (PMSR), Clinical Effects Analysis Report (CEA) from the scratch! Knowing this skills will set you apart from the crowd and you can easily land you six-figure jobs in a reputed medical device companies.
You will be well versed in rules, regulations and standards for medical device industries across the world such as ISO14971 (Risk Management, ISO) , ISO13485, ISO 22000, 21CFR810 (FDA, USA), RDC 40/2015 (Anvisa, Brazil), EU 2017/745 MDR, MHLW MO 169 (PMDA, Japan) and TGR 2002 (TGA, Austrialia) and be able to talk about them in meetings, interviews and even with auditors.
You will learn how processes like complaint reviews, Corrective and Preventative Maintenance (CAPA), Process Validation (PV), Equipment Qualification (IQ/OQ/PQ) ties into Risk Management and why companies need to do these activities.
Whether you are just getting into Risk Management, or wants to freshen up your skills, or are looking to advance your career in the field, this course can provide you with more than enough knowledge to be the top 5% in your organization.
Note: This course is directly related to and applies to Enterprise Risk Management (ERM). Medical device and Pharmaceutical companies are an enterprises themselves so this course teaches you everything you need to know to manage risks in an enterprise. In fact, you will learn risk management in so much in-depth from this course that you can tackle risk management activities on any enterprise.
Note: This course also ties into Financial Risk Management (FRM)Â as we go in depth on the types of risks companies deals with, and financial risk is one of them. Hence students wanting to learn FRMÂ can also greatly benefit from this course.
Who this course is for:
- Professionals looking to be promoted or looking to get into medical device, pharmaceutical industry and food industry
The most extensive course on Risk Management available in Udemy!
This course is designed to teach you everything that you need to know to become a Subject Matter Expert (SME) in Risk Management and Quality Management System (QMS) within Medical device, pharmaceutical industry and any other enterprise that conducts risk management activity.
Regardless of your current skills and experience, after taking this course, you will be comfortable communicating to experts in medical device industry about Quality management systems (QMS) and risk management (RM)Â standards and regulations.
By the end of this course you will be a pro at creating complex Risk Assessment and Risk Measurement documents such as PFMEA, DFMEA, HACCP, HAZOP, Benefit-Risk Analysis, Post Market Surveillance Report (PMSR), Clinical Effects Analysis Report (CEA) from the scratch! Knowing this skills will set you apart from the crowd and you can easily land you six-figure jobs in a reputed medical device companies.
You will be well versed in rules, regulations and standards for medical device industries across the world such as ISO14971 (Risk Management, ISO) , ISO13485, ISO 22000, 21CFR810 (FDA, USA), RDC 40/2015 (Anvisa, Brazil), EU 2017/745 MDR, MHLW MO 169 (PMDA, Japan) and TGR 2002 (TGA, Austrialia) and be able to talk about them in meetings, interviews and even with auditors.
You will learn how processes like complaint reviews, Corrective and Preventative Maintenance (CAPA), Process Validation (PV), Equipment Qualification (IQ/OQ/PQ) ties into Risk Management and why companies need to do these activities.
Whether you are just getting into Risk Management, or wants to freshen up your skills, or are looking to advance your career in the field, this course can provide you with more than enough knowledge to be the top 5% in your organization.
Note: This course is directly related to and applies to Enterprise Risk Management (ERM). Medical device and Pharmaceutical companies are an enterprises themselves so this course teaches you everything you need to know to manage risks in an enterprise. In fact, you will learn risk management in so much in-depth from this course that you can tackle risk management activities on any enterprise.
Note: This course also ties into Financial Risk Management (FRM)Â as we go in depth on the types of risks companies deals with, and financial risk is one of them. Hence students wanting to learn FRMÂ can also greatly benefit from this course.
Who this course is for:
- Professionals looking to be promoted or looking to get into medical device, pharmaceutical industry and food industry
User Reviews
Rating
Anil Sharma Kandel
Instructor's Courses
Udemy
View courses Udemy- language english
- Training sessions 30
- duration 4:31:23
- Release Date 2023/06/12